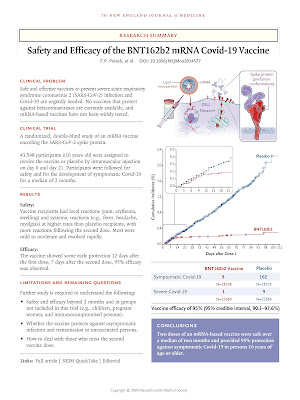
Title: Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine Authors: Fernando P. Polack, M.D., Stephen J. Thomas, M.D., Nicholas Kitchin, M.D., Judith Absalon, M.D., Alejandra Gurtman, M.D., Stephen Lockhart, D.M., John L. Perez, M.D., Gonzalo P.rez Marc, M.D., Edson D. Moreira, M.D., Cristiano Zerbini, M.D., Ruth Bailey, B.Sc., Kena A. Swanson, Ph.D., Satrajit Roychoudhury, Ph.D., Kenneth Koury, Ph.D., Ping Li, Ph.D., Warren V. Kalina, Ph.D., David Cooper, Ph.D., Robert W. Frenck, Jr., M.D., Laura L. Hammitt, M.D., .zlem Türeci, M.D., Haylene Nell, M.D., Axel Schaefer, M.D., Serhat .nal, M.D., Dina B. Tresnan, D.V.M., Ph.D., Susan Mather, M.D., Philip R. Dormitzer, M.D., Ph.D., Uğur Şahin, M.D., Kathrin U. Jansen, Ph.D., and William C. Gruber, M.D., for the C4591001 Clinical Trial Group N Engl J Med 2020 Dec 10. doi: 10.1056/NEJMoa2034577. Online ahead of print. Background Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the resulting coronavirus diseas...